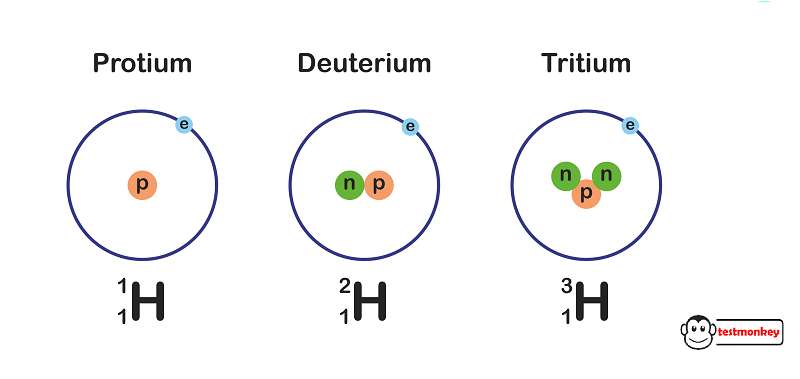
Hydrogen has three isotopes: protium (1H), deuterium (2H), and tritium (3H). Protium is the most common isotope and has no neutrons, deuterium has one neutron, and tritium has two neutrons.
Details about the isotopes of hydrogen :
- Protium (1H): Protium is the most common and stable isotope of hydrogen. Its nucleus contains one proton and no neutrons. It is sometimes called “ordinary hydrogen” and accounts for more than 99.98% of naturally occurring hydrogen on Earth.
- Deuterium (2H): Deuterium is an isotope of hydrogen that is also stable but less abundant than protium. Its nucleus consists of one proton and one neutron, making it twice as massive as protium. Deuterium is often used in nuclear research, as a tracer in chemical reactions, and in some industrial applications.
- Tritium (3H): Tritium is a radioactive isotope of hydrogen. Its nucleus contains one proton and two neutrons, making it much heavier than both protium and deuterium. Due to its radioactive nature, tritium undergoes beta decay with a half-life of approximately 12.3 years. It is used in some special applications such as self-luminous exit signs and in some types of nuclear weapons.
These isotopes of hydrogen play an important role in various scientific and industrial fields due to their unique properties.
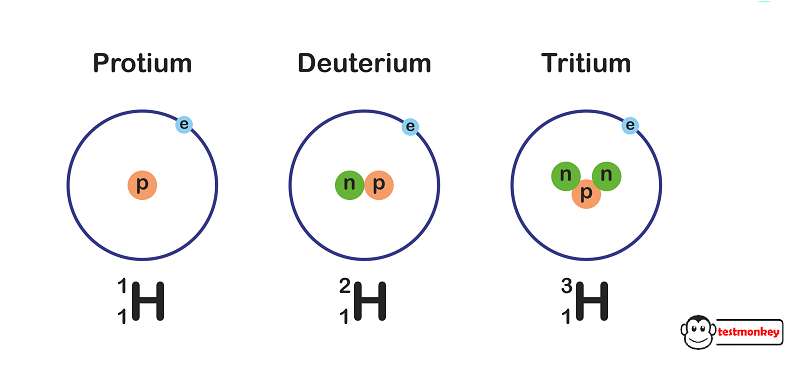
Some applications of different isotopes of hydrogen:
Protium (1H):
Protium is the most common form of hydrogen and is used in various chemical reactions and industrial processes. It is an important component in the production of ammonia, which is used in fertilizers and other chemical products.
Protium is also used in hydrogen fuel cells to generate electricity in a clean and environmentally friendly way.
Deuterium (2H):
Deuterium is used in scientific research and as a tracer in various industrial processes. By incorporating deuterium into molecules, scientists can track chemical reactions and study the behavior of molecules in different environments.
Heavy water, which contains deuterium oxide (D2O), is used as a neutron moderator in some types of nuclear reactors. This helps to slow down neutrons to maintain a controlled nuclear reaction.
Tritium (3H):
Tritium is used in the production of self-luminous exit signs and various types of luminous paint. In nuclear research, tritium can be used as a radioactive tracer to study biochemical and physiological processes.
Tritium can be used in nuclear weapons as part of the fusion process, although its use is strictly controlled and heavily regulated.
Overall, the different isotopes of hydrogen have diverse applications in scientific research, energy production, and various industrial processes. While protium and deuterium are stable and non-radioactive, tritium’s radioactive nature makes its applications more specialized and requires careful handling and regulation.

About the Author
Manish love to write and he is a Civil Servant. Users can follow Manish on Instagram 
Rao Chandra Sen of Marwar (1562-81)
Emperor Rao Chandra Sen of Marwar ruled with glory from 1562 to 1581. His steadfast…
What is Deep Underground Neutrino Experiment – DUNE ?
A large-scale international research project, the “Deep Underground Neutrino Experiment” (DUNE) aims to study neutrinos,…
What is the Charter of the United Nations?
The United Nations Charter, signed in San Francisco on June 26, 1945, and ratified on…
अफ्रीका का बँटवारा और उपनिवेशवाद (Colonialism in Africa) [PART-II]
अफ्रीका का बँटवारा और अफ्रीका में Kris Bryant calls Astros’ sign stealing ‘worse than steroids’…
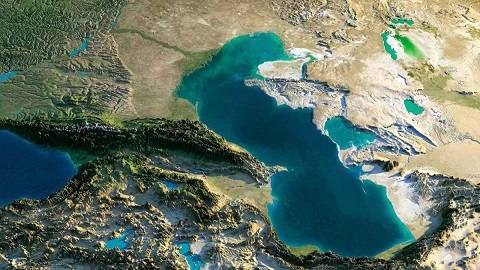
कैस्पियन सागर समुद्र और झील दोनों क्यों है?
कैस्पियन सागर (Caspian Sea) पांच देशो के तटों को जोड़ता है या सीमाओं से लगता…
The Falkland dispute / war
The Falkland Islands have been the subject of a territorial dispute between Argentina and the…